Hyperinflammatory syndrome in a paediatric patient with a recent ... - BMC Infectious Diseases
A 4-year-old female Caucasian child was admitted to the emergency department with fever and acute respiratory failure. The personal and familial anamnestic recall brought no elements of suspicion for a past SARS-CoV-2 infection. The chest X-ray and subsequent computed tomography (CT) showed multiple and bilateral ground glass areas and patchy consolidations in the inferior lobes, pneumomediastinum with supraclavicular and cervical bilateral subcutaneous emphysema (Fig. 1). The microbiological assessment on broncho-alveolar lavage (BAL) was positive for Pneumocystis jiroveci (PJ) and galactomannan, SARS-CoV-2 proved negative. As her respiratory dynamics progressively deteriorated, she was intubated and assisted through mechanical ventilation.
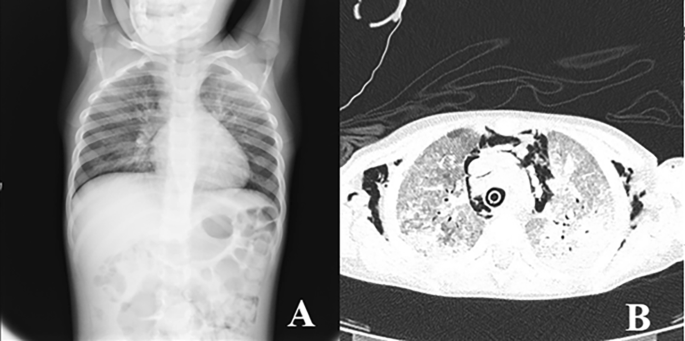
Chest X-Ray (A) and CT (B) showing multiple and bilateral ground glass areas and patchy consolidations in the inferior lobes, pneumomediastinum
At the anamnestic recall the parents reported a history of recurrent respiratory infections since she was 3 years old, a previous episode of ocular HSV infection and recurrent oral thrush. Due to the patient's medical history and the evidence of PJI and pulmonary aspergillosis, an immunological assessment was performed, and a severe CD4-penia emerged: CD4+ was 1.06% (6 cell/µl, normal value 500–1000). Soon after the diagnosis of HIV-positivity was finalised with a viral load of 83.429 copies/ml. She was classified as a stage 3, according to Centres for Disease Control (CDC) classification system for HIV infection [11]. Combined ART was initiated at the diagnosis of HIV infection, with a lamivudine, zidovudine and lopinavir/ritonavir; alongside treatment for PJ and aspergillosis was started with Trimethoprim/Sulfamethoxazole, Caspofungin and Ambisome.
The microbiological assessment run to investigate possible coinfections proved positivity for CMV (31,446 copies/ml) and EBV (8542 copies/mL). Also, at the oral cavity inspection, the patient presented some vesicles positive for HSV. Acyclovir and Gancyclovir were then added to her therapeutic regimen. On the 51st day after she had started ART, she started presenting fever with a progressive worsening of clinical conditions: no other microbiological agents were isolated at the analysed samples (blood, stools and urine) and there was no improvement with broad-spectrum antibiotic therapy.
Her laboratory assessment showed progressive trilinear cytopenia (lowest values: haemoglobin 7,7 g/dl, absolute neutrophil count 690 cells/mcl, platelet count 14.000 cells/mcl), progressive increase of C-reactive protein (up to 4,28 mg/dl), hyponatremia (serum sodium 129 mEq/l), hypoalbuminemia (3,1 g/dl) and hypofibrinogenemia (76 mg/dl ). Triglycerides were slightly increased (160 mg/dl) and ferritin levels were increased (up to > 12.000 ng/ml). Cardiac enzymes showed progressive elevation (high sensitivity troponin up to 40,9 pg/ml and proBNP 1548 pg/ml). At that time her HIV viral load was undetectable and CD4+ cell count was 35 cells/mcl (normal value 630–2110). We have always studied the expansion trend of expansion of CD4+ cell in relation to CD8+ cell, we also evaluating the expression of CD45 RA+RO− (naïve) and CD45RA−RO+ (memory) on the T cells: these analyses were compatible with the success of ART (Fig. 2).
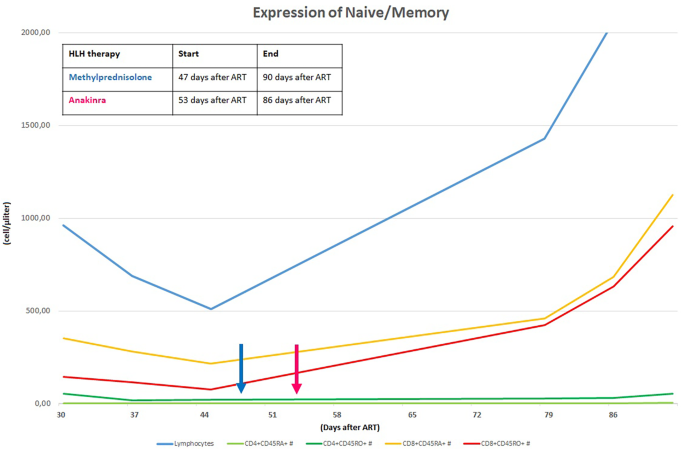
CD4+ and CD8+ RA/RO progression after ART initiation
In order to assess the differential diagnosis between HLH and IRIS, T-cell activation was investigated through the HLA-DR+ and CD38+ evaluation on CD4+ lymphocytes, which resulted always less than 1/microliter (Fig. 3). In the suspicion of HLH and in order to assess other causes of cytopenia, a bone marrow aspirate and biopsy were performed: evidence of bone marrow cytopenia (Fig. 4) with prevalence of T-cells and macrophages with signs of phagocytosis was found. The immune activation markers, HLA-DR + and CD38+, were present on the CD8+ lymphocytes (Fig. 5), making the diagnosis of HLH even more suggestive [12]. We could not assess soluble IL2-R at that time in our hospital.
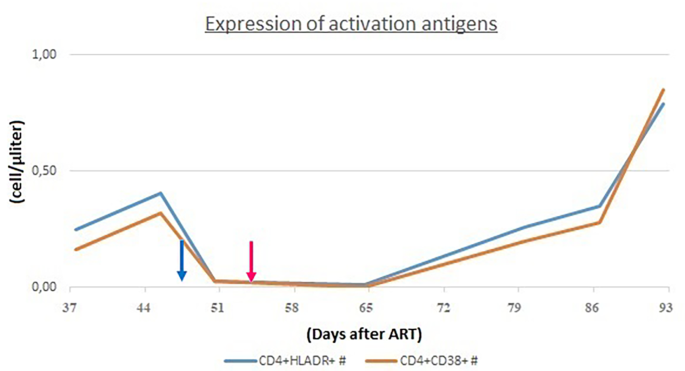
CD4+ cells did not show immune activation markers (HLADR+ and CD38+)
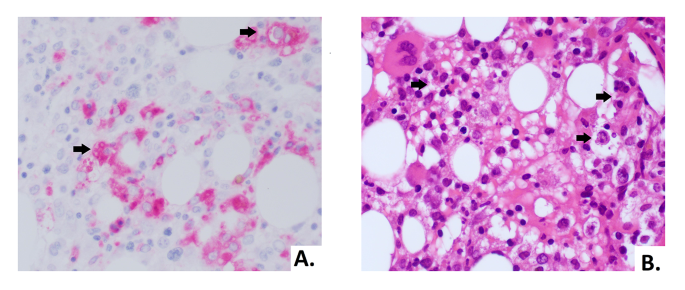
The biopsy showed a bone marrow with low cellularity, marked reduction of erythroid line, predominantly T lymphocytosis and histiocytosis with aspects of hemophagocytosis (arrows). (A) Morphology of histiocytes in CD68 immunohistochemical staining (40X). (B) Hematoxylin and eosin stain of bone marrow biopsy with hemophagocytosis (40X)

CD8+ cells showed immune activation markers (HLADR+ and CD38+)
Due to the presence of six diagnostic criteria [3] the diagnosis of HLH was made: persistent fever > 38,5 °C, cytopenia involving more than two lineages, hypertriglyceridemia and hypofibrinogenemia, splenomegaly, hyperferritinemia and hemophagocytosis in bone marrow. Furthermore, Patient's NK showed a lower degranulation after stimulation with K562 cells than healthy donor (Fig. 6). However due to an ongoing treatment with systemic corticosteroids such assay can only be partially considered reliable for degranulation.
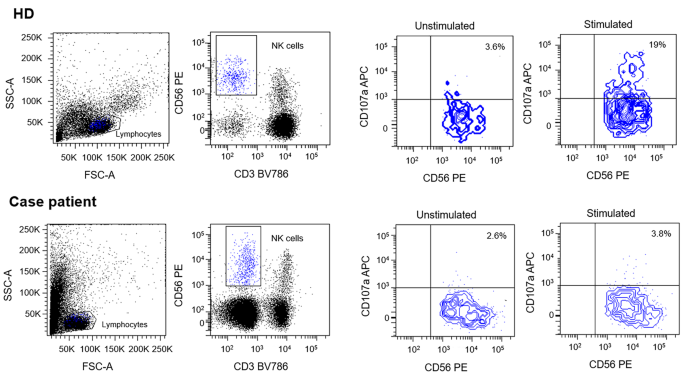
In order to evaluate the expression of CD107a and its relative effect on NK cells degranulation, Healthy Donor (HD) and patients'-derived Peripheral Blood Mononuclear Cells (PBMC) were isolated from EDTA-blood samples. Specimens were diluted in Phosphate Buffered Saline (PBS) solution and layered over Ficoll 1.077 g/ml. Then, 2 × 106 cells/cm2 were plated on cell culture dishes in RPMI medium containing 50 U/ml penicillin, 50 mg/ml streptomycin, 2 mM L-glutamine and 10% Fetal Bovine Serum (FBS). Human IL-2 (100U/mL) was added to allow cells expansion. Cells were incubated overnight at 37 °C. The day after, PBMC were co-cultured with or without lymphoblast K562 target cells at E:T ratio 1:1 for 3 h at 37° C. Cells were then stained with anti-human CD3, CD56 and CD107a antibodies. Results were evaluated by comparing CD107a expression between PBMC with or without co-cultured K562 cells. Data were acquired with a FACS LSRFortessa (Becton Dickinson, USA). Flow cytometer profiles were analyzed using FACSDiva Software (Becton Dickinson, USA). Patient's NK showed no degranulation after stimulation with K562
Then a glucocorticoid therapy with three pulses of methylprednisolone (30 mg/kg/day) was started. The patient returned upyretic after the first pulse of methylprednisolone. After the three pulses, she was started on dexamethasone (10 mg/m2/day) as maintenance therapy. The laboratory assessment showed a progressive improve of the inflammatory parameters with worsening cytopenia and coagulopathy.
In consideration of the insufficient response to glucocorticoid therapy, the treatment was implemented with intravenous interleukin-1 receptor antagonist (Anakinra, 100 mg twice a day = 14 mg/kg/day).
After the treatment with anakinra was started, the patient's clinical conditions and laboratory parameters showed a progressive improvement. Glucocorticoid therapy was progressively reduced and the interleukin-1 receptor antagonist was initially reduced to 7 mg/kg/dose (100 mg once per day) after 21 days of treatment. Anakinra was reduced by 25% after 5 days; after 3 days, the dose was reduced by 30% and eventually stopped after an additional 24 h of treatment. After the immunosuppressive therapy was stopped, the patient maintained good clinical condition and normalization of inflammatory markers.
Comments
Post a Comment