Overcoming barriers to patient adherence: the case for developing ... - Nature.com
Abstract
Poor medication adherence is a pervasive issue with considerable health and socioeconomic consequences. Although the underlying reasons are generally understood, traditional intervention strategies rooted in patient-centric education and empowerment have proved to be prohibitively complex and/or ineffective. Formulating a pharmaceutical in a drug delivery system (DDS) is a promising alternative that can directly mitigate many common impediments to adherence, including frequent dosing, adverse effects and a delayed onset of action. Existing DDSs have already positively influenced patient acceptability and improved rates of adherence across various disease and intervention types. The next generation of systems have the potential to instate an even more radical paradigm shift by, for example, permitting oral delivery of biomacromolecules, allowing for autonomous dose regulation and enabling several doses to be mimicked with a single administration. Their success, however, is contingent on their ability to address the problems that have made DDSs unsuccessful in the past.
Introduction
More than half of the world's population takes at least one drug each day, and the demand for pharmaceuticals is only expected to increase as the global disease burden continues to grow1. The benefits that a drug seemingly affords in a highly controlled setting, however, will not translate to real-world use if patients do not take their medication as prescribed. Poor medication adherence is the most common reason for disparities observed between results obtained in randomized clinical trials (RCTs) and real-world outcomes2,3 and remains pervasive; estimates of non-adherence are around 50% for chronic illnesses4,5. In the United States alone, poor adherence is responsible for an estimated 125,000 deaths per year, a figure comparable with the number of deaths caused by colorectal cancer, breast cancer and prostate cancer combined6,7. Poor adherence is also estimated to cause 10% of all hospitalizations and underlie $100–300 billion of avoidable health-care costs annually owing to wasted medicine, unnecessary diagnostic procedures and excessive health-care provider utilization8,9,10. Rates of non-adherence are especially high among older people, who are more likely to require complicated treatment plans and suffer from cognitive and/or functional impairments (for example, dysphagia) that impede their ability to administer certain types of medication11. Owing to a globally ageing population and a worldwide shift in the general disease burden from acute to chronic conditions, the adverse effects of non-adherence are only expected to increase12.
The key reasons for poor adherence are patient forgetfulness, anxiety about treatment-associated adverse effects, low motivation due to a perceived lack of efficacy, poor health literacy and aversion to the health belief model, and stigmatization4,8. Other factors that may play a role include high prescription costs and insufficient patient–provider communication. Besides negatively impacting the health of an individual, pervasive non-adherence can have a pernicious effect on the health of a community, especially as it pertains to communicable diseases. For example, failing to complete a vaccination schedule or a course of antibiotics or antivirals as prescribed can lead to the emergence of a resistant strain of a contagious bacteria or virus. Vaccine refusal has been implicated in outbreaks of varicella, measles and pertussis, among others13.
Improving adherence is recognized as one of the most impactful and cost-effective strategies for improving the health of the general population, yet it has not garnered the same attention as other approaches for improving wellness4. A mere 1% increase in drug utilization among individuals enrolled in Medicare and Medicaid in the United States is estimated to result in a $3 billion reduction in national health-care spending (0.2% of the total Medicare and Medicaid budget in 2020 (ref. 14))15,16. Traditional health-care provider-mediated strategies for improving adherence by educating and empowering patients have produced inconsistent and often underwhelming results17 (Box 1). These interventions are often too complex, requiring health-care infrastructure and/or some extent of personalization, to be cost-effective at scale. Drug delivery systems (DDSs) are promising technological alternatives that can mitigate the logistical factors negatively impacting real-world adherence. DDSs are formulations, systems or technologies used to modulate the release of a drug in the body over time and/or target the drug to a particular tissue or cell type. The first DDS, a sustained-release system delivering dextroamphetamine, was approved in 1952 (Box 2). A timeline describing the development of several key DDSs — with an emphasis on those that did (or are expected to) improve medication adherence — is provided in Fig. 1.
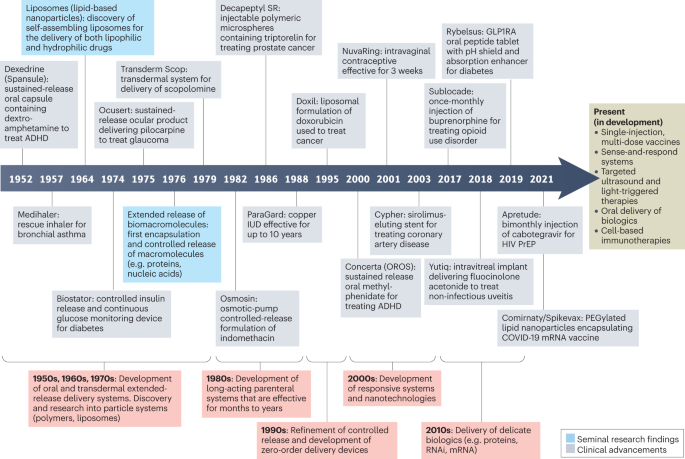
This timeline illustrates examples of several key delivery technologies and drug delivery systems (DDSs) developed between the 1950s and the 2020s, many of which have improved or are expected to improve patient adherence. Several technologies currently in development that may be featured in future DDSs are also included. ADHD, attention deficit hyperactivity disorder; GLP1RA, glucagon-like peptide 1 receptor agonist; HIV, human immunodeficiency virus; IUD, intrauterine device; PrEP, pre-exposure prophylaxis.
In this Review, we first overview the fundamentals of DDSs and the mechanisms by which they can improve adherence. We then summarize the impact that DDSs have had on patient adherence across four disease and intervention types: chronic, relapsing–remitting, acute and prophylactic. Finally, we discuss the lessons that can be learned from several novel DDSs that failed to realize broad commercial success.
Overview of drug delivery systems
Controlled-release DDSs can be classified by numerous characteristics including their route of medication administration (for example, oral, transdermal, intravenous, intramuscular, subcutaneous, transmucosal; Fig. 2), the device type (for example, injectable microparticle depot, extended-release oral formulation, intravaginal ring) or the drug release profile afforded by the system (pulsatile, first-order, sustained, zero-order or stimuli-responsive).
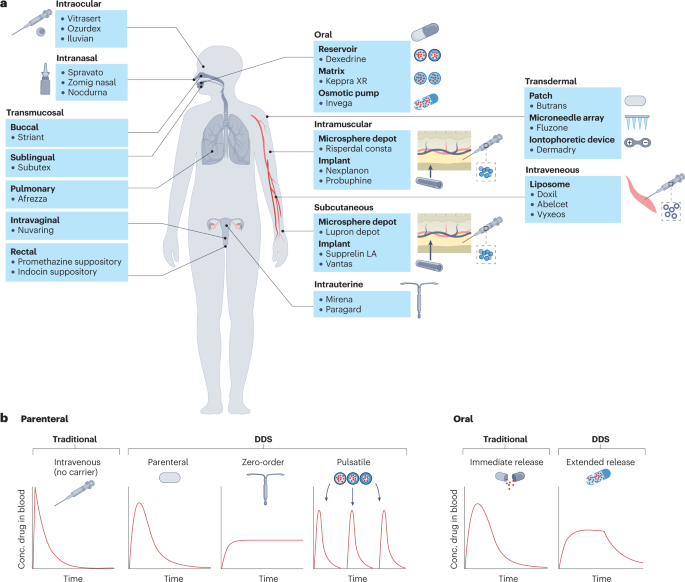
a, US Food and Drug Administration (FDA)-approved examples of drug delivery systems (DDSs) grouped by route of administration (oral, intramuscular, transdermal, subcutaneous, intraocular, intranasal, intrauterine and transmucosal (pulmonary, sublingual, buccal, intravaginal and rectal)). b, Pharmacokinetic profiles showing the plasma concentration of a drug following a single dose, based on the type of release. Traditional, non-DDS formulations of parenteral and oral drugs result in rapid clearance of drug from the blood, whereas some DDSs can prolong the duration over which the drug concentration remains within the therapeutic window without increasing the peak drug concentration. In the case of pulsatile release, DDSs can also allow for multiple, pre-programmed release events mimicking bolus doses of drug following a single administration.
DDSs are especially useful when the active pharmaceutical ingredient (API) has dose-limiting side effects, a narrow therapeutic window and/or a short half-life that makes maintaining the proper drug concentration difficult. Examples of DDSs that have been developed to address these issues include the liposomal formulation of the cardiotoxic chemotherapeutic doxorubicin (Doxil), a subcutaneous injectable microparticle suspension of somatotropin, a protein with a half-life of 20–30 min following intravenous injection18 (Nutropin Depot) and an extended-release oral formulation of the anticonvulsant drug phenytoin, which has a therapeutic index of only two (Phenytek capsules)19. In other instances where the payload is too fragile to survive in the body over therapeutically relevant timescales without a suitable carrier (for example, nucleic acids), a DDS such as a lipid-based nanoparticle may be required.
Chemical modifications and microenvironment modulation are two additional paradigms for improving the pharmacokinetics of an API. The former entails changing the physicochemical properties of a drug to create a new molecular entity20,21, and the latter entails changing the immediate vicinity of the drug to increase its solubility, stability and/or modulate the resulting immune response22. Although these additions may be included as part of a DDS formulation, they do not, by themselves, meet the definition of DDS used in this Review. Instead, we focus on platform technologies that can be applied to more than one API.
Limitations
Formulating APIs in DDSs is not a one-size-fits-all approach, however, and there are limitations common to certain classes of DDSs that are worth noting. For example, surgically implanting a device requires an invasive procedure and, in some instances, frequent monitoring by a health-care professional. This is also true for some state-of-the-art DDSs in preclinical development, including responsive particle systems that require external stimuli such as ultrasound or focused light to release cargo in a targeted manner, or systems that utilize instrument-mediated modes of cell transfection for gene therapy (that is, electroporation or biolistic (gene gun) delivery). Some classes of DDSs may be more likely to malfunction than their traditional alternative(s) owing to added device or usage complexity. Certain devices may also be less accessible owing to limited demand, scant coverage by insurance providers, a lack of enabling infrastructure, limited awareness among patients and providers, and/or costly premiums, especially in low and middle-income countries. These drawbacks, however, are arguably true of all nascent technologies and should diminish as further development and cost optimization enables broader adoption. Finally, some patients may express apprehension or outright refusal in favour of traditional, 'tried and true' methods of medication administration, depending on factors such as the severity of their disease and the device's route of administration and usability23.
Design considerations
Generally, there are several key design considerations that DDS development should abide by depending on the device's intended disease target(s). Given that patients with chronic conditions are likely to use it on a frequent (often, daily) basis, reliability, affordability and ease of use generally take priority. Factors including the size of the device (if used externally), the ease of administration and the severity of rapidly onset side effects, if any, can affect its perception among patients and its clinical utility. For relapsing–remitting conditions, it may additionally be beneficial to design a device capable of accommodating medically recommended changes in treatment owing to variable disease progression. The design considerations for DDSs used to administer prophylactic medication are similar to those for DDSs intended for use in patients with chronic and relapsing–remitting conditions, given the similarity in dosing duration requirements; however, device discretion may additionally carry more weight for some prophylactic drugs, such as pre-exposure prophylaxis (PrEP) and contraceptives. Where applicable, all non-surgically implanted DDSs intended for long-term use would benefit from monitoring capabilities to track patients' adherence and the device effectiveness. The design considerations for DDSs used to treat acute conditions include those mentioned above, but there may be additional considerations given the time-sensitive nature of the condition (as is the case with an acute infection). These may include the speed of drug delivery and the portability of the device.
Improving patient acceptability and adherence
DDSs can improve the pharmacokinetics of an API and/or enable alternative delivery routes, potentially allowing for a reduction in dosing frequency and/or abatement of adverse effects. Some DDSs can also allow for added discretion, benefitting patients who feel embarrassed by having to regularly store and take pills. These improvements, among others, can enable patients to overcome barriers to adherence such as forgetfulness, premature discontinuation and stigma-related aversion. Below, we discuss seven major ways in which DDSs can improve adherence.
Reduced dosing frequency
Patients prefer to take a drug less often and are, accordingly, more adherent when their treatment regimen aligns with their preferences24,25,26,27,28. There is strong evidence to support a significantly higher rate of adherence to drugs taken once daily versus those taken multiple times per day for various conditions, including bisphosphonates (BPs) for osteoporosis, angiotensin-converting enzyme inhibitors for hypertension and sulfonylureas for type 2 diabetes (T2D), among many others. On a more granular level, there is evidence to support both the notion of an inverse, monotonic relationship between dosing frequency and adherence29 as well as a subtle or insignificant difference between adherence rates for drugs taken multiple times per day30,31. In one meta-analysis, adherence rates to oral medications used to treat chronic diseases across three definitions of adherence (taking, regimen and timing; Box 1) were found to be progressively lower for regimens requiring administration of two, three and four doses per day compared with once-daily dosing regimens, with the disparity growing more pronounced as the stringency of the adherence definition increased30. In another analysis, adherence rates among patients with asymptomatic chronic diseases taking once-daily medications were significantly higher compared with rates among those taking twice-daily or thrice-daily medications26.
In contrast to immediate-release formulations such as capsules and intravenous injections, extended-release DDSs release a drug over a longer period of time, enabling less frequent dosing. Three common types of extended-release formulation, either for oral delivery or parenteral implantation/injection, are matrix, reservoir and osmotic-controlled systems (Fig. 3). Each system is capable of achieving delivery times of 12–24 h (if taken orally) or, potentially, years (if implanted parenterally) but is subject to trade-offs, including the rate and precision of drug release and ease of manufacturing. Some reservoir systems additionally consist of multiple types of particle, each made of biodegradable polymers of a different composition and/or thickness that allow for burst release of a drug at different times — a so-called pulsatile DDS32. These systems constitute a promising platform for addressing adherence issues with multi-dose vaccines. These are often associated with low rates of completion due, in part, to the burden of visiting a health-care provider multiple times, an adherence barrier that is heightened in low-resource settings33,34.
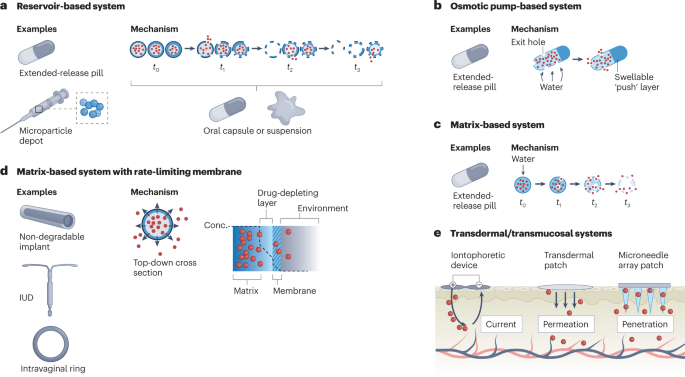
a, Reservoir-based systems. These consist of a hollow, drug-filled core encapsulated by a degradable polymer. Over time, the polymeric shell degrades in a composition and thickness-dependent manner to release the drug. Using multiple types of shell allows for a fraction of the drug to release at a certain time. This technology can be used in capsules or as part of a microparticle depot suspension in oral and parenteral delivery systems, respectively. b, Osmotic pump-based systems. These systems are often used for extended-release oral delivery and consist of a water-permeable, insoluble polymer laden with drug and, often, a so-called expandable 'push' layer encapsulated in a hard coating. Exit holes are drilled through the coating to expose the polymer to the outside. Over time, water infiltrates the capsule and causes both layers to expand, pushing drug out through the exit holes. c, Matrix-based systems. These systems consist of drug embedded within a water-permeable, soluble monolithic matrix. Over time, water infiltrates the matrix (often, in the form of a tablet) and causes it to degrade, enabling drug release. These types of system are most often used in extended-release oral formulations. d, Matrix-based systems with a rate-limiting membrane. These consist of a drug-laden matrix core surrounded by a semi-permeable membrane that limits the rate of drug release, enabling pseudo zero-order delivery. This technology is often found in non-degradable, long-lasting implants, such as intrauterine devices (IUDs) and intravaginal rings. e, Transdermal/transmucosal systems. The three types of system shown here are iontophoretic devices, transdermal patches and microneedle array patches. Iontophoretic devices achieve delivery by creating an electric field that efficiently shuttles charged moieties across the skin barrier. Transdermal patches allow for passive delivery of molecules smaller than 500 Da. Finally, microneedle array patches penetrate the skin to deliver drug directly into the cutaneous layer.
Other extended-release DDSs include near zero-order delivery systems such as intravaginal rings, osmotic pumps, actuated pumps and implantable microchips, which are capable of providing a steady rate of drug release over a period ranging from hours to years35. Finally, nano-formulations, such as liposomes and dendrimers, can help extend the circulating time of a drug or improve its deposition characteristics, thereby increasing the therapeutic duration of the drug and reducing its required dosing frequency.
Avoidance of first-pass metabolism and accelerated onset of action
The bioavailability of oral drugs is limited by hepatic first-pass metabolism, leading to variability in the rate and extent of absorption. DDSs that avoid first-pass metabolism by using a parenteral route — such as intravenous, subcutaneous, intramuscular, transdermal, intranasal, sublingual or buccal administration — to deliver a drug directly into the bloodstream or the target site enable comparatively less material to achieve the same therapeutic effect in a well-controlled manner. They also enable a faster onset of action compared with oral delivery, which can be crucial for adherence; if patients do not immediately feel a lessening of their symptoms, they may stop taking their medication before it has a chance to exert its intended effect.
Intranasal DDSs enable a drug to access the brain via the olfactory or trigeminal nerves, bypassing hepatic first-pass metabolism, harsh gastrointestinal conditions and the blood–brain barrier to elicit the desired therapeutic effect within minutes instead of hours36,37. So-called nose-to-brain delivery systems are typically used to deliver classes of drugs such as anti-seizure medications, migraine medications, cholinesterase inhibitors and antidepressants38,39.
Rapid improvement of depressive symptoms is critical in patients with severe depression who are acutely suicidal. Accordingly, studies have shown that the short-term effects of antidepressants are predictive of long-term results40,41. Esketamine is an anaesthetic drug that is used to treat patients with treatment-resistant depression and has a quicker onset of action than traditional antidepressants. The oral bioavailability of esketamine, however, is only 8–11% and the rate of absorption appears to vary considerably between patients, possibly owing to factors such as stomach contents and gut motility42. In those taking oral esketamine, significant changes are often detected only 2–6 weeks after treatment initiation43. Intranasal esketamine (Spravato), in comparison, has a much higher bioavailability (46–54%) and is fast-acting, reaching a peak plasma concentration 20–40 min after dosing and lessening depressive symptoms as quickly as 4 h after the first dose.
Mitigation of concentration-dependent adverse effects
Experiencing adverse effects or anxiety about potential adverse effects is a major deterrent to patient adherence44,45,46. A high plasma concentration of certain drugs, including glucagon-like peptide 1 receptor agonists (GLP1RAs), cholinesterase inhibitors and BPs, immediately after dosing is directly correlated with the onset of gastrointestinal adverse effects such as nausea, diarrhoea and vomiting. Accordingly, these side effects are reported less frequently by patients taking long-acting formulations of these drugs than those taking short-acting formulations47,48,49,50. For patients with epilepsy, taking consecutive doses of immediate-release antiepileptic drugs (many of which have short half-lives and narrow therapeutic indices51) results in large peak-to-trough fluctuations, which may increase the risk of both seizures and concentration-dependent toxicity52. Extended-release formulations of antiepileptic drugs such as phenytoin (Dilantin) and valproate (Depakote ER), in comparison, are associated with improved tolerability, offer significant improvements in quality of life and can mitigate the effects of missed or delayed doses53.
Besides short-term adverse effects, there are other risks associated with repeated exposure to high concentrations of some drugs. These include an increased risk of developing resistance (as is the case with some antibiotics and antivirals) or developing tolerance and physical dependence (as is the case with opioids)4,54. Extended-release formulations have the potential to mitigate many of these issues by minimizing the peak-to-trough fluctuations in plasma drug concentration, enabling it to stay below toxic levels and within the therapeutic window. Because the drug metabolism rate is typically a function of concentration, these formulations have the added benefit of enabling less total drug to achieve the same therapeutic duration, potentially reducing the burden on the liver and kidneys.
Lowered barrier to continued use
Long-acting DDSs can offer a lowered barrier to continued use, benefitting patients who would otherwise prematurely stop taking a medication for the various reasons discussed below.
Patients taking fast-acting drugs may begin to feel better within a short period of time after initiating a course of medication and, considering their problem solved, fail to continue taking it as a result. This phenomenon is prevalent during the remission phase of various relapsing–remitting diseases, such as inflammatory bowel disease (IBD), relapsing–remitting multiple sclerosis and asthma. Premature discontinuation is also common in patients taking a course of antibiotics for an acute infection, which may increase the risk of developing antibiotic resistance.
Patients may also prematurely stop treatment if taking a drug with a delayed onset of action, as is common for antidepressants, antipsychotics and some immunosuppressants. In the case of depression, premature medication discontinuation contributes to its undertreatment and is a risk factor for developing a treatment-resistant form of the disease55.
Finally, patients may prematurely discontinue a drug because of its short-term side effects. Some treatment regimens for chronic diseases, such as interferon therapy in the treatment of multiple sclerosis or chronic hepatitis C, often result in flu-like symptoms that typically diminish over time56,57. The initial, acute onset of these symptoms is a deterrent to patient acceptability58, with the number and severity of symptoms inversely correlated to adherence59,60,61. Patients who are taking naltrexone for a substance use disorder can also initially experience unpleasant withdrawal symptoms during the first phase of treatment that can last for up to 2 weeks, depending on the substance. This often leads to premature discontinuation, a phenomenon that is common among patients receiving treatment for a substance use disorder62.
Reduced pain
Needle phobia is estimated to affect one out of every five people, and those afflicted are more likely to avoid medical treatment involving needles, including vaccination63,64. This fear is estimated to be the primary reason for non-compliance with recommended paediatric immunization schedules in 7–8% of cases65 and may account for as many as 10% and 16% of all individuals expressing COVID-19 (ref. 66) and influenza vaccine hesitancy63, respectively.
Nasal and oral vaccines are alternatives to vaccines delivered via intramuscular injection with a hypodermic needle. In a survey of parents whose children had received vaccines via both intranasal administration and intramuscular injection, a significantly larger percentage found the intranasal formulation to be more well tolerated and generally regarded it as more favourable67. However, oral vaccines are limited by challenges associated with oral delivery, such as withstanding the harsh conditions of the gastrointestinal tract and achieving sufficient absorption across the intestinal mucosal barrier despite a short residence time68. For these reasons, although they can provide mucosal immunity and are highly acceptable to patients (≥90%), oral vaccines are often unable to confer systemic immunity69.
Transdermal microneedle array patches (MAPs) are a type of DDS in preclinical development that deliver drugs to the epidermis or upper dermis, avoiding the cutaneous pain receptors found in the lower dermis and allowing for painless drug delivery. Injections with micro-sized needles are also less likely to cause serious skin irritation, redness or swelling and present a lower risk of infection than standard intramuscular injections70. Studies have shown that patient acceptability of MAPs is high, with 70–90% reporting that they would prefer to use a MAP rather than receive an intramuscular injection with a hypodermic needle71,72,73,74.
Increased cost-effectiveness
High out-of-pocket medication costs are a deterrent to patient adherence, especially in resource-limited settings75,76,77,78. DDSs have the potential to reduce the total amount of drug required to achieve a therapeutic effect by controlling the drug's systemic concentration and rate of clearance. From a financial perspective, this benefit might be greatest for controlled-release DDSs delivering costly biologics. For example, anti-VEGF biologics are highly effective in the treatment of ocular diseases such as macular degeneration and macular oedema but are relatively expensive, and lowering the out-of-pocket costs of these treatments has been shown to significantly improve adherence79. Intravitreal bolus injections of the anti-VEGF treatments ranibizumab and aflibercept are annually estimated to use 160 and 190,000 times more drug, respectively, than that which a zero-order DDS would require80. If cost was to scale linearly with dose size, this translates to an estimated annual saving of approximately $10,000 for both treatments when formulated in a DDS, which is likely to far exceed the fabrication costs of such a device81. Moreover, controlling release over an extended duration could reduce the number of times a physician needs to administer the drug, requiring fewer doctor's visits and reducing costs.
MAPs can also achieve dose sparing of vaccines, often requiring only 1–10% of the antigenic material that would be required in a subcutaneous or intramuscular injection due to the efficient activation of skin-resident antigen-presenting cells82,83. They may also have the potential to be stored outside the cold chain and/or self-administered, further lowering costs and improving accessibility.
Destigmatization
Stigma plays an important role in treatment acceptability and adherence for various conditions, including neuropsychiatric disorders, epilepsy, attention deficit hyperactivity disorder (ADHD) and human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS)84,85,86,87,88.
Patients with ADHD may need to take as many as eight immediate-release methylphenidate tablets a day. This can be a source of stigma and embarrassment, especially among children who need to take one or more doses during the school day88,89. Several extended-release formulations of methylphenidate have been developed, including the osmotic controlled-release oral delivery system Concerta XL. This DDS, which consists of an immediate-release coating of methylphenidate encapsulating an extended-release osmotic pump system, produces a quick onset of action and avoids the rapid development of tolerance, extending the drug's duration of efficacy to 10–12 h. Accordingly, results from RCTs suggest that this system leads to higher satisfaction and treatment adherence among patients over traditional immediate-release formulations90,91.
Individuals taking antiretrovirals for HIV/AIDS prophylaxis or therapy face a unique combination of stringent adherence requirements92,93 and a high rate of stigmatization, leading them to prefer comparably invasive, long-acting DDSs (for example, injectable suspensions and intravaginal rings) over short-acting oral drugs and pericoital microbicides94,95,96. In a survey of individuals receiving oral antiretroviral therapy, more than four fifths reported that they would definitely or probably try an injectable long-acting extended-release formulation if the dosing frequency was reduced to once monthly or better97. Accordingly, the two long-acting injectable antiretroviral therapies recently approved by the FDA have both been shown to be more effective in clinical trials over an oral PrEP alternative, ostensibly due to a demonstrable improvement in patient adherence98,99,100.
Trends in adherence by disease type
Adherence rates and auspicious DDS intervention strategies vary by disease type. In this section, we discuss the effects that implementation of a DDS and/or treatment modification attainable with a DDS have had on adherence in four categories of diseases and treatments: chronic, relapsing–remitting, acute and prophylactic interventions. These four categories broadly encompass most conditions that are currently treatable/addressable using DDSs and that substantially contribute to disability-adjusted life years lost worldwide. For example, cancer, diabetes, hypertensive heart disease and major depressive disorder alone (all discussed below) were collectively responsible for an estimated 15% of disability-adjusted life years lost in 2019 (refs. 101,102,103,104). In each section, we begin by discussing the impact that clinically approved DDSs have had on patients afflicted with that particular disease type and, where applicable, conclude with a brief discussion of promising DDSs in preclinical development. For further reference, a comparison of traditional versus DDS/long-acting formulations for the same API is provided in Table 1.
Chronic disorders
Adherence rates across chronic conditions are variable, but generally straddle 50%4. Across several conditions including hypertension, hypercholesterolaemia and heart disease, the risk of mortality in non-adherent patients is approximately twice that of adherent patients105 and rates of adverse outcomes are 1.5-fold to 5.4-fold higher106.
The potential of DDSs to improve adherence in patients with chronic conditions depends on the characteristics of the disease, the established treatment regimen and the administration parameters of the DDS. Given the low-level persistent nature of diseases such as osteoporosis, glaucoma and hypertension, long-acting injectables or implants might effectively promote adherence. In patients with a disease that can be well managed but for which no long-acting treatments currently exist, such as T2D, a DDS capable of making a seminal advancement (for example, enabling oral delivery or autonomous function) may be necessary in order to achieve widespread adoption. By contrast, for patients living with an often-debilitating disease with few treatment options, such as cancer, marginal improvements are much more meaningful and patients may be more willing to tolerate a DDS that produces adverse effects in exchange for added efficacy. For those with neuropsychiatric disorders, faster time to onset, a reduced barrier to continued use (usually in the form of an injectable or implantable DDS) and increased discretion are key characteristics.
Diabetes
Diabetes differs from many other chronic conditions in that it currently requires very frequent self-monitoring and intervention. Accordingly, poor adherence levels in patients with T2D are associated with an increased risk of hospitalization, complications, cerebrovascular disease and death107.
Several insulin analogues are commonly prescribed to patients with T2D: rapid-acting (lispro, aspart and glulisine), short-acting (regular human insulin), intermediate-acting (neutral protamine hagedorn) and long-acting (detemir and glargine). Each offers a trade-off between their onset and duration of action108. Although there is little information regarding the differences in rates of adherence between patients using different types of insulin, there is evidence that patients are more satisfied with rapid-acting analogues owing to the increased flexibility of dosing109.
Non-insulin oral and injectable antidiabetic agents, such as biguanides, sulfonylureas and GLP-1RAs, are also prescribed to some patients. There are multiple formulations of GLP-1RAs taken via subcutaneous injection twice daily, once daily or once weekly110. Persistence rates among patients with T2D taking a once-weekly GLP-1RA formulation are consistently reported to be higher than for those taking once-daily or twice-daily formulations, likely owing, in part, to a lower incidence of nausea and vomiting111,112,113,114.
Inhalable insulin was developed in the late 1990s and early 2000s as a non-invasive alternative to subcutaneous injected insulin. In clinical testing, inhalable insulin was found to have comparable efficacy and a quicker onset of action compared with subcutaneously injected insulin115. Owing to the ease of administration and convenience, patie...
Comments
Post a Comment