Polyamine metabolism impacts T cell dysfunction in the oral mucosa ... - Nature.com
Abstract
Metabolic changes in immune cells contribute to both physiological and pathophysiological outcomes of immune reactions. Here, by comparing protein expression, transcriptome, and salivary metabolome profiles of uninfected and HIV+ individuals, we found perturbations of polyamine metabolism in the oral mucosa of HIV+ patients. Mechanistic studies using an in vitro human tonsil organoid infection model revealed that HIV infection of T cells also resulted in increased polyamine synthesis, which was dependent on the activities of caspase-1, IL-1β, and ornithine decarboxylase-1. HIV-1 also led to a heightened expression of polyamine synthesis intermediates including ornithine decarboxylase-1 as well as an elevated dysfunctional regulatory T cell (TregDys)/T helper 17 (Th17) cell ratios. Blockade of caspase-1 and polyamine synthesis intermediates reversed the TregDys phenotype showing the direct role of polyamine pathway in altering T cell functions during HIV-1 infection. Lastly, oral mucosal TregDys/Th17 ratios and CD4 hyperactivation positively correlated with salivary putrescine levels, which were found to be elevated in the saliva of HIV+ patients. Thus, by revealing the role of aberrantly increased polyamine synthesis during HIV infection, our study unveils a mechanism by which chronic viral infections could drive distinct T cell effector programs and Treg dysfunction.
Introduction
How viruses alter the metabolic states of immune cells and the precise mechanisms underlying the persisting immune dysfunction during chronic viral infections are key questions that have not been fully addressed. Given that discrete tissue microenvironments govern diversity, function, and plasticity of immune cells that in turn regulate anti-viral responses and inflammatory signals, devising effective and targeted approaches require a comprehensive understanding of tissue-specific immunology. In the era of combined anti-retroviral therapy (cART), HIV infection is characterized by systemic inflammation, persisting mucosal immune cell dysfunction, and increased risk of biological aging and associated comorbidities. People living with HIV (PLWH) represent ~37.7 million [30.2–45.1 million] of the world population with a significant compromise on the quality of life and no current treatments for co-morbidities in PLWH1. Higher prevalence of periodontitis, oral candidiasis, and cancers are observed in PLWH, although the underlying mechanisms are less studied2,3. Oral mucosal T helper (Th) dysregulation and hyperactivation coincide with enhanced toll like receptor (TLR) and NLR family pyrin domain containing (NLRP)3-driven inflammasome signaling, elevated levels of FOXP3+Tregs and their dysfunction in PLWH4. Our previous study has shown that HIV infection associated Th dysregulation involved an enrichment of dysfunctional FOXP3+ cells (TregDys) defined by PD-1hiIFN-γ+FOXP3+CD4+ markers. Although higher expression of PD-1 and IL-1β contributed to TregDys induction and proliferation, the precise mechanism of overall Th dysregulation in PLWH is unclear. Polyamine synthesis represents one of the fundamental processes and has recently been shown to determine the functional fates of Th subsets5,6,7. Ornithine decarboxylase (ODC-1) is the rate-limiting enzyme in polyamine biosynthesis pathway and converts L-ornithine to the first polyamine putrescine, which is sequentially converted into spermidine and spermine8. Spermidine acts as a substrate in the process of hypusination of eukaryotic translation initiation factor 5A (EIF5A), which is upregulated during T cell receptor (TCR) activation and regulates cytokine expression in Th cells5. EIF5A hypusination is a post-translational modification of a specific lysine residue that requires two consecutive enzymatic steps involving deoxyhypusine synthase (DHPS), and deoxyhypusine hydroxylase (DOHH). These components of polyamine synthesis pathway were recently implicated in altering Th activation, cytokine expression, and cellular fidelity5.
Here we show that HIV-mediated alterations in polyamine metabolism contribute to the mechanism of tilting the balance between Th17 and Treg cell subsets in the oral mucosa of PLWH. Our previous study characterized the TregDys subset during HIV infection and showed that TregDys induction and expansion requires caspase-1 activity and IL-1β release4. Here we found that pyroptotic loss of Th17 cells coupled with the skewing of the Th balance towards TregDys phenotype also requires ODC-1 mediated polyamine synthesis and EIF5A hypusination, which are also enhanced during HIV infection. ODC-1 and polyamines do not directly regulate Th17 cell frequencies in the context of HIV infection. However, blockade of ODC-1 activity as well as EIF5A hypusination using N1-guanyl-1,7-diaminoheptane (GC7), a spermidine analog reduced the frequency of TregDys during HIV infection in vitro. The addition of exogenous polyamines also induced amphiregulin (AREG) upregulation and proliferation of TregDys. Importantly in PLWH, increased Treg/Th17 ratio and CD4 hyperactivation positively correlated with the upregulation of polyamine putrescine in the oral mucosa. In conclusion, our study uncovers the role of polyamine metabolism as a cardinal determinant of Th dysregulation and chronic inflammation in the context of viral diseases.
Results
RNA-sequencing of oral gingival mucosa and salivary metabolome analysis show dysregulation of arginine and polyamine pathways in PLWH
Using global metabolomic profiling of saliva combined with transcriptional profiling of the oral gingival mucosa, we first identified metabolic alterations in cART treated PLWH versus uninfected controls. We combined the transcriptomic and metabolomics datasets using a combined computational approach that annotates gene expression changes associated with alterations in metabolite pool sizes. Linked gene/metabolite associations were subsequently mapped to pathways using MetaboAnalyst and Metascape. Metabolite identifications were made with Compound Discoverer 3.1 SP1 and MZmine2 software. MS1 and MS2 spectra were matched using a mass tolerance of m/z = 0.1. The raw data were acquired and aligned using the Compound Discover based on the m/z value and the retention time of ion signals. Ions from both ESI− or ESI+ were merged and imported into the SIMCA-P program (version 14.1) for multivariate analysis. Further data processing was performed by using DecoID (DecoID v0.3.0) to deconvolute chimeric MS2 spectra and increase the identification rate. Metabolite identifications were made with level 2 confidence according to the Metabolomics Standards Initiative9. Metabolite changes were considered significant if they reached a threshold of Log2FC = 1, p < 0.05. Using these metabolite and gene lists, we examined pathways significantly altered by HIV infection (Group B; n = 40) compared to uninfected controls (Group A; n = 26). The enrichment analysis indicated a widespread metabolic reprogramming within the oral cavity associated with HIV infection. Partial Least-Squares Discriminant Analysis (PLS-DA) and Variable Importance in Projection (VIP) score plots are shown in Supplementary Fig. 1. Alterations in metabolite classes included amino acids, energy metabolites, lipids, and carbohydrates. Together, these changes were reflected in the top enriched pathway hits that include amino acid utilization (arginine and proline, tryptophan, and branched-chain amino acids), arachidonic acid metabolism, and the use of carbohydrates (Fig. 1A, B). Activated immune cells have previously been shown to upregulate glucose utilization to support proliferation, differentiation, and effector functions such as cytokine secretion10. Therefore, we examined the changes in expression of glycolytic genes in PLWH versus controls. Enzymes that control glycolytic flux including phosphofructokinase (PFKFB3) are strongly upregulated by HIV infection (Log2FC 2.68, p = 1.15 × 104) (Supplementary Fig. 2). Several transcriptional regulators of hypoxic and glycolytic responses and responses to glucose deprivation such as hypoxia-inducible factor 1 (HIF-1)α and DNA damage-inducible transcript 4 protein (DDIT4) were also enriched in PLWH in gingival mucosa. Interestingly, glucose was also increased in PLWH while the immediate downstream intermediates glucose 6-phosphate and fructose 6-phosphate were decreased compared to healthy controls (Supplementary Fig. 3). However, no significant changes in glycolytic and TCA metabolites, or lactate were noted in PLWH (Supplementary Fig. 3). Arachidonic acid is also a key lipid mediator driving inflammatory responses11,12. Our transcriptomic and metabolomic datasets were significantly enriched for hits in this pathway (Supplementary Fig. 2). Therefore, we examined enzymes and metabolites involved in the production of eicosanoids and leukotrienes within the oral mucosa. Arachidonic acid is significantly increased (p = 0.049) in saliva along with the eicosinoids, 5-hydroperoxyeicosatetraenoic acid (5-HPETE), and 12-hydroxyeicosatetraenoic acid (12-HETE)13. Increased levels of 5-HPETE and 12-HETE were also consistent with the upregulation of their biosynthetic enzymes arachidonate 5-lipoxygenase (ALOX5) and CYP4F3 detected in the transcriptomic analysis. Moreover, we detected perturbations in the tryptophan/ kynurenine axis in PLWH. This pathway consumes tryptophan to produce molecules such as kynurenine that regulate immune cell function14. Kynurenine also functions as a ligand for aryl hydrocarbon receptors, the activation of which promotes Treg differentiation15. Although heightened tryptophan catabolism and kynurenine levels would be consistent with the Treg enrichment that we showed previously in PLWH4, we found an opposite trend, including upregulation of kynureninase, moderately lower levels of kynurenine, and increased levels of tryptophan in saliva in oral mucosa of PLWH (Supplementary Fig. 2).
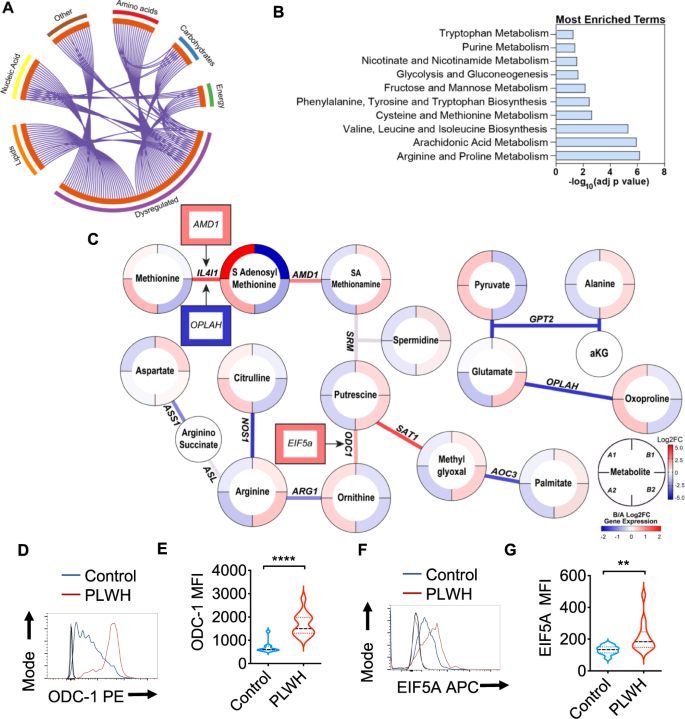
Metabolite levels were determined by LC-MS in saliva samples from healthy controls (Group A) and PLWH (Group B) (uninfected controls n = 26; PLWH; n = 40). Genes are mapped to pathways (Kegg, GO, Reactome), and the algorithm calculates the fold change and p-value between how many hits were input and how many hits would be expected from random chance. Log10 adjp-value indicates the significance of each term compared to random chance, larger values indicate higher likelihood that the pathway is truly enriched. Pathways are analyzed individually with no comparisons made. A Circos plot linking genes significantly dysregulated between groups A and B (purple outer ring) to their associated KEGG pathways via purple line. Graph was generated via Metascape. B Overall the most enriched terms by -log10p value were generated using dysregulated genes and metabolites via Metaboanalyst 5.0. C Genes and metabolites associated with the most enriched term, Arginine proline metabolism. Circles represent metabolites ringed by Log2FC (PLWH vs Controls). Lines represent enzymatic genes colored by Log2FC. Rectangles indicate genes not directly involved in enzymatic reactions but are upstream or downstream of the linked metabolites, ringed by Log2FC. Metabolite changes were considered significant if they reached a threshold of Log2FC = 1, p < 0.05. Cells from gingival mucosa were processed for flow cytometry and stained for intracellular ODC-1 (Control n = 10; PLWH n = 13) (D, E) and EIF5A (Control n = 10; PLWH n = 14) (F, G). Light and dark gray histograms represent staining controls for Control and PLWH cells respectively. Flow cytometry analysis was conducted gating on CD4+ T cells. E and G show the respective statistical analysis of geometric mean fluorescence intensity (MFI). Median values ± SEM are plotted. (Mann–Whitney U test ****P < 0.0001; **P = 0.007; Two-tailed). Circles within box plots represent each replicate.
Based on the pathway enrichment analysis data, we further examined other pathways that may be related to immune activation in the oral mucosa. We subsequently examined gene/metabolite relationships driving the alterations in amino acid metabolism by mapping the dysregulated genes to associated metabolites (Log2FC cutoff =1) using metabolomics computational tools, including MetaboAnalyst and DecoID16. From this analysis, we identified key nodes altered by HIV infection that are involved in the production and use of amino acids including arginine (Fig. 1C). To determine age-dependent changes in control (Group A) and PLWH (Group B), we stratified the groups based on their age (A1 and B1 aged <60 and A2 and B2 age >60). HIV infected individuals showed significant downregulation of several enzymes involved in glutamate utilization or production, including 5-oxoprolinase (OPLAH, Log2FC = −1.64, p = 0.016), and alanine aminotransferase (GPT2, Log2 FC = −1.64, p = 0.007) independent of age-stratification (Fig. 1C). Reduced expression of GPT2 impacts the production of alanine from pyruvate and is consistent with alterations in the activity of this enzyme, alanine levels were significantly increased, while pyruvate is decreased in the saliva of PLWH. Interestingly, individuals with HIV showed increases in arginine in saliva while the pool size of citrulline was decreased compared to healthy controls (Fig. 1C). This led us to examine nitric oxide and ornithine pathways. Several enzymes including two enzymes that play a role in polyamine synthesis; spermidine/spermine N(1)-acetyltransferase (SAT1) and ornithine decarboxylase (ODC-1) show increased transcription in PLWH. The precursor of polyamines, ornithine is also decreased in PLWH, while ODC-1, the polyamine putrescine and EIF5A transcript downstream to putrescine were elevated relative to healthy controls indicating that there may be increased polyamine synthesis in the microenvironment of the oral mucosa (Fig. 1C). Because ODC-1 was recently shown to instruct Th subset fates5,17, we hypothesized that HIV-induced altered metabolic states could impact Treg/Th dysregulation in oral mucosa of PLWH4. Consistent with the upregulation of ODC-1 and EIF5A transcripts in PLWH (Fig. 1C), flow cytometry analyses also revealed heightened expression of these proteins in CD4+ T cells of PLWH individuals when compared to uninfected controls (Fig. 1D–G; gating strategy; Supplementary Fig. 4). Altogether, the oral mucosa of HIV infected patients provided an important insight into how polyamine metabolism may be relevant to altering T cell functions in mucosae.
HIV-1 infection causes Treg/Th17 ratio skewing
Next, we postulated that HIV-induced Treg dysfunction in PLWH4 might involve the polyamine pathway. We employed the oral lymphoid system of TCR-activated human tonsil organoid cultures (HTOC) in vitro5. First, we examined ODC-1 expression in different TCR-stimulated CD4+ subsets in non-polarized and uninfected HTOC. ODC-1 is known to be upregulated in TCR-activated cells and highly expressed in in vitro-polarized Th1 and Th2 cells, but reduced in Th17 cells and Tregs5,6,7. We compared CD25+ activated vs non-activated cells, IFN-γ+ Th1 cells vs IFN-γneg non Th1 cells, ROR-γt+IL-17A+ Th17 cells vs ROR-γtnegIL-17Aneg non Th17 cells, CD4+CD25+FOXP3+Tregs vs CD4+CD25+FOXP3neg non Tregs, and CD4+CD25+FOXP3+PD-1+IFN-γ+ TregDys, vs CD4+CD25+FOXP3+PD-1 negIFN-γneg non TregDys, in TCR-stimulated HTOC. CD25+ cells, Th1, Th17, and Tregs expressed higher ODC-1 levels compared to their counterparts (Supplementary Fig. 5). Consistent to a recent study5, Th1 cells showed increased ODC-1 expression than Tregs and Th17 cells. Our results also showed that TregDys expressed significantly lower ODC-1 than non TregDys. These data suggested distinct differences in polyamine metabolism between CD4+ T cell subsets (Supplementary Fig. 5) and provided a rationale to examine polyamines in CD4+ T cells in the context of HIV infection. As shown previously, about 3- 7% HIV-GFP+ cells were observed upon HIV infection of these cultures4,18. HIV-1 infection causes significant depletion of IFN-γ and IL-17A+ effector Th subsets and results in the induction and expansion of dysfunctional PD-1+IFN-γ+FOXP3+ cells (TregDys)4,18. Because of the known role of Th17 cells in mucosal integrity, and the previous evidence of dysregulation of mucosal Treg/Th17 ratios in acute SIV infections19, we examined Treg/Th17 ratios in HIV-1-infected HTOCs. As the markers we used distinguished the subsets with relevance to ODC-1 expression (Supplementary Fig. 5), we employed the same to identify Th17 cells in HTOC. To assess the effects of HIV and anti-retroviral inhibitor (ARI) alone in cultures, we had these control cultures. To mimic HIV infection followed by anti-retroviral treatment in patients (Fig. 1), we added ARI efavirenz 24–36 h after HIV infection and allowed the cells to expand with ARI for 6–7 days. Similar to our previous findings4,18, Tregs showed significant cell death and were restored with ARI, efavirenz (Fig. 2A, top). After confirming TregDys expansion during HIV infection that persisted in the presence of ARI (Fig. 2A, bottom), we assessed the frequency of Th17 cells by determining the expression of ROR-γt, CCR6, and IL-17A in CD4+ T cells. The cell numbers of Th subsets were also determined in these cultures (Supplementary Fig. 6A). Our results showed that HIV-1 infection led to a significant loss of Th17 cells and the ARI treatment post-HIV infection was not able to restore them in vitro (Fig. 2B, C). Alterations in the frequencies of these two subsets resulted in significant enhancement of TregDys/Th17 ratios during HIV infection and were sustained even after the ARI treatment (Fig. 2D). These results prompted us to determine the role of polyamines during HIV infection in the presence of ARI.
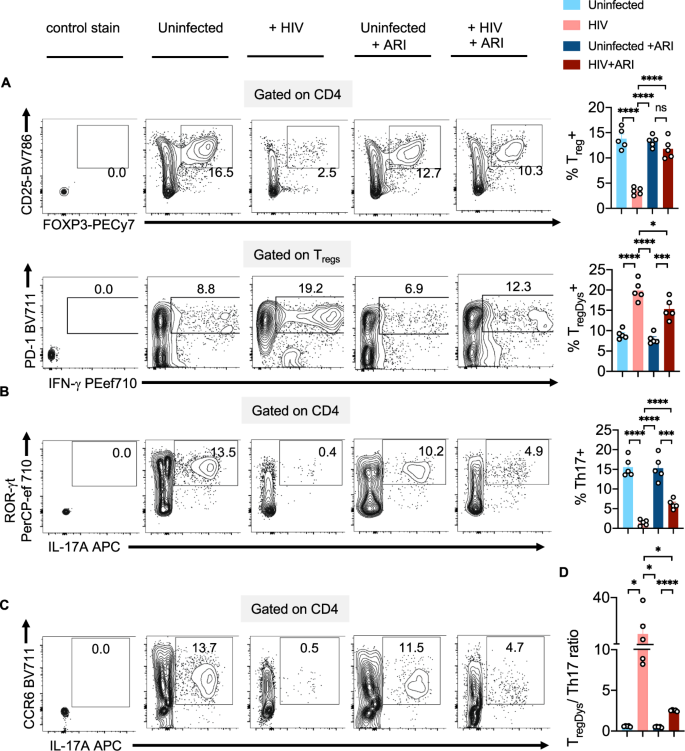
TCR-stimulated HTOC was infected with HIV. Control cultures were not infected (Uninfected). In some cultures, ARI was added the next day to block subsequent rounds of infection. Cells were allowed to expand in the presence of TGF-β1 (10 ng/ml) and IL-2 (100 U/ml) for 6 days. A FOXP3 and CD25 expression (top) and PD-1 and IFN-γ expression (bottom) in CD4+ (CD3+CD8neg) FOXP3+ cells, showing the frequency of Tregs and TregDys. Expression of IL-17A and ROR-γt (B), and IL-17A and CCR6 (C) in CD4+ cells, showing the frequency of Th17 cells. Representative contour plots (left), statistical analyses of proportions of the cells from 5 independent experiments showing the frequency of these subsets (A, B, right). D Statistical analysis of TregDys/Th17 ratio based on the results in A and B. Results derived from five independent experiments are presented as mean values +/− SEM. A–D ****P < 0.0001; ***P < 0.0002; *P < 0.02; Two-tailed; Unpaired t test. Circles within box plots represent each replicate.
HIV-1 upregulates ODC-1, NLRP3, EIF5A, EIF5A hypusination and polyamine synthesis in CD4+ T cells even in the presence of ARI
To examine polyamine metabolism, we first focused on ODC-1 expression during HIV-1 infection in vitro. ARI was added 24–36 h after HIV infection and the cells were allowed to expand for 6–7 days. Consistent with the up-regulation of mRNA levels of ODC1 and EIF5a in PLWH oral mucosa, we found that HIV-infected cells showed higher levels of this protein in the presence of ARI (Fig. 3A). Then we measured the expression of EIF5A and its hypusination that occurs downstream of ODC-1 activity (Fig. 3B). The expression of both EIF5A and hypusinated EIF5A were significantly upregulated in HIV-1-infected CD4+ cells in HTOC (Fig. 3C, D). HIF-1α is a metabolic checkpoint protein that can be induced by non-hypoxic stimuli such as TCR- and PI3-K pathways, which result in divergent functions in the pathogenesis of inflammatory diseases20. Given the increases in HIF-1a mRNA expression in PLWH (Supplementary Fig. 2), and the known roles of HIF-1α and polyamines in orchestrating Th lineage functions5,6,7,21, we speculated that HIF-1α may be linked to polyamine metabolism. However, our results showed that HIV-1 did not upregulate HIF-1α expression in CD4+ T cells in vitro (Supplementary Fig. 6B). Fluorimetry assay of polyamines showed that intracellular polyamines were also significantly elevated in CD4+ T cells in HIV infected cultures (Fig. 3E). By measuring polyamine levels in the supernatants of HIV infected cultures, we confirmed that increased intracellular polyamines was not due to decreased export to extracellular media (Supplementary Fig. 7). In summary, these results show that Th cells expanding in the presence of ARI post-HIV infection upregulate polyamine biosynthesis. These findings in oral mucosal lymphoid tonsillar cultures recapitulated the increased polyamine metabolism observed in the oral mucosa of HIV+ patients on therapy (Fig. 1A).
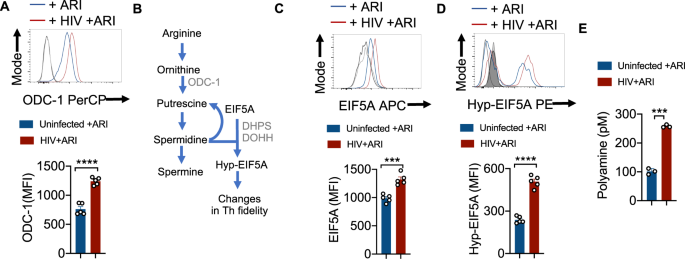
A HTOC were TCR-activated and infected with HIV. ARI was added the next day to block subsequent rounds of infection. Cells were allowed to expand in the presence of TGF-β1 (10 ng/ml) and IL-2 (100 U/ml) and ARI for 6 days as described in methods (n = 5 independent experiments). Representative flow cytometric data showing ODC-1 expression in CD3+CD8 negative cells (CD4+ T cells) (top) and statistical analyses of MFI from five independent experiments (bottom) are shown. B Key intermediate steps in polyamine synthesis. Cells were stimulated and processed for flow cytometry as in A for determining the expression of EIF5A (C) and hypusinated EIF5A in CD4+ T cells (D). Light and dark shaded gray histograms represent staining controls for EIF5A (C) and hypusinated EIF5A (D) respectively. Mean values +/− SEM; ****P < 0.0001; ***P < 0.0002; Two-tailed; Unpaired t test in A–D. E Fluorometric polyamine estimation in cellular lysates from cells stimulated as in A for 4 or 7 days (n = 3 independent experiments). Polyamine concentrations were normalized to cell numbers. Mean values +/− SD; ***P = 0.0002; Two-tailed; Welch's t test. Circles within box plots represent each replicate.
HIV-1 activated caspase-1 and IL-1β are required for ODC-1 upregulation in CD4+ T cells
Previous studies by us and others have demonstrated the augmentation of NLRP3 signaling, activation of caspase-1 and elevated IL-1β expression in CD4+ T cells during acute and chronic HIV infection4,22,23. Based on ODC-1's ability to regulate NLRP3 expression in macrophages24, and NLRP3's function in activating caspase-125, we hypothesized that NLRP3 may also be upregulated in HIV-infected HTOC CD4+ T cells. We confirmed that HIV-1 infection significantly induced NLRP3 in HTOC CD4+ T cells expanding in the presence of ARI (Supplementary Fig. 8). We then interrogated whether NLRP3/IL-1β signaling is involved in HIV-induced ODC-1 upregulation in the presence of ARI. IL-1β promoted the upregulation of ODC-1 in TCR- stimulated CD4+ T cells (Fig. 4A). Moreover, ODC-1 expression significantly correlated with the expression of IL-1Rα (CD121) on CD4+ T cells and was diminished by Anakinra, the IL-1β signaling inhibitor (Supplementary Fig. 9, Fig. 4B, C). To further elucidate the function of caspase-1 mediated cleavage and activation of IL-1β in inducing ODC-1, we employed VX-765 the caspase-1 inhibitor, and Anakinra. We infected the purified CD4+ cells from TCR stimulated HTOC, infected with HIV-1 in the presence or absence of inhibitors, and treated them with ARI, 24–36 h post-infection. Flow cytometry analysis revealed that both VX-765 and Anakinra significantly downregulated ODC-1 expression in HIV-1-infected cells(Fig. 4D). Neither NLRP3 inhibition nor HIF-1α inhibition directly modulated ODC-1 expression or increase in TregDys in HIV-infected cultures (Supplementary Fig. 10A, 10B). While Caspase-1 and IL-1β blockade did not affect Treg frequencies, they significantly decreased the TregDys frequencies (Fig. 4E, Supplementary Fig. 11). On the other hand, Caspase-1 inhibition through VX-765 partially restored Th17 cell frequencies, but blocking of IL-1β did not impact HIV-1 mediated Th17 loss (Supplementary Fig. 11, Fig. 4F). Further analysis showed that Th17 cell loss was due to the cell death triggered by HIV-1, only partially restored by ARI (Supplementary Fig. 12). Caspase-1 involvement in Th17 cell loss suggests that cell death is pyroptotic. These data indicate that during HIV infection, caspase-1 is critical to 1) IL-1β release and TregDys increase by ODC-1 up-regulation and 2) Th17 cell loss through pyroptotic cell death. Together, caspase-1 and IL-1β drive the TregDys/Th17 skewing during HIV infection (Fig. 4G). These data position caspase-1 and IL-1β at the juncture of polyamine pathway dysregulation, cell death mechanism, and Th fate alteration induced by HIV-1.
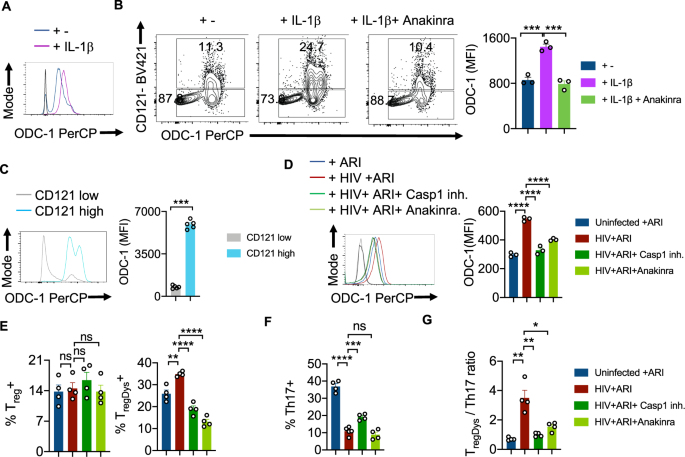
HTOC cells were TCR stimulated with IL-1β (10 ng/ml) for 6 days in the presence or absence of Anakinra (10 μg/ml). A Histogram plot showing ODC-1 expression. B ODC-1 and CD121(IL-1R1) expression in CD4+ T cells (n = 3 independent experiments). C ODC-1 expression gating on CD121 high versus CD121 low cells (n = 5 independent experiments). Flow cytometry plots of unstained and FMO stain controls are provided in the Supplementary Fig. 9. D–G Purified tonsillar CD4+ T cells were TCR activated, infected with HIV and allowed to expand for 6-days post-infection in the presence of ARI, Efavirenz (50 nM). Caspase-1 inhibitor (VX-765, 250 nM) and Anakinra (10 μg/ml) were added as indicated, 36 h post-infection. ODC-1 expression (D), Treg and TregDys proportions (n = 3 independent experiments) (E), Th17 proportions (n = 4 independent experiments) (F), and TregDys/Th17 ratios (n = 3 independent experiments) (G) are shown. Light and dark gray histograms represent staining controls for ARI and HIV+ ARI conditions, respectively in A and D. Flow cytometry plots of these respective graphs are provided in Supplementary Fig. 11. Results are derived from three to four independent experiments and are presented as mean values +/− SEM. ****P < 0.0001; ***P = 0.0001–0.0002; **P < 0.002; *P 0.002–0.02; Two-tailed; Unpaired t test in B–G. Circles within box plots represent each replicate.
HIV infection mediated Th dysregulation is dependent on ODC-1
To confirm that ODC-1 activity underlies polyamine synthesis and Treg dysregulation caused by HIV-1, we infected HTOC CD4+ T cells in the presence or absence of ODC-1 inhibition using Difluoromethylornithine (DFMO) (ODC-1 inhibitor I), N-(4-Pyridoxyl)-Ornithine(BOC)-OMe (POB)(ODC-1 inhibitor II), and ODC-1 shRNA lentiviral particles. Using the polyamine fluorometric assay, we first confirmed that ODC-1 inhibition diminishes intracellular polyamines (Fig. 5A). Consistent with the requirement of ODC-1's activity in promoting EIF5A hypusination, inhibition of ODC-1 significantly downmodulated the expression of both EIF5A and hypusinated EIF5A in HIV-1 infected CD4+ cells in HTOC (Fig. 5B, C, Supplementary Fig. 13). As expected, ARI restored the Treg levels to uninfected, but ODC-1 inhibition further decreased Tregs moderately (Fig. 5D, top, 5F). While HIV-1 infection boosted the frequency of TregDys, blockade of ODC-1 activity significantly diminished TregDys to the levels in uninfected cultures (Fig. 5D, bottom, F). ODC-1 inhibition did not affect the Th17 depletion caused by HIV, (Fig. 5E, F), but it significantly restored the TregDys/Th17 ratios to the uninfected levels in CD4 T cells (Fig. 5G). These findings strengthen the idea that ODC-1 is required for the enrichment of intracellular T cell polyamine levels and Th fate skewing caused by HIV-1.
HTOC CD4+ T cells were TCR activated and infected with HIV. ARI (Efavirenz; 50 nM), DFMO (ODC-1 inhibitor I; 2.5 mM), POB (ODC-1 inhibitor II; 100 μM), Control shRNA lentiviral particles-A with polybrene (2 μg/ml), and ODC1 shRNA lentiviral particles with polybrene (2 μg/ml) were added as indicated, 36 h post-infection. A Fluorometric polyamine estimation in cell lysates on day 6 post- infection. n = 3 independent experiments; Mean values +/− SD; **P < 0.003; ****P < 0.0001; Two-tailed; Welch's t test. B Statistical analysis of EIF5A expression (MFI), and C Statistical analysis of hypusinated EIF5A expression (MFI) from three experiments. Flow cytometric overlays are provided in Supplementary Fig. 13. D Contour plots showing FOXP3 and CD25 (Tregs) expression in CD4 cells (above) and PD-1 and IFN-γ (TregDys) in FOXP3+cells (below). E Contour plots showing ROR-γt and CCR6 expression (Th17) in CD4 cells. Quantification of Treg, TregDys, Th17 (F), and TregDys/Th17 ratios (G) based on the data from D and E. Data are representative of four (n = 4) independent experiments and are presented as mean values +/− SEM. ****P < 0.0001; ***P = 0.0001; **P < 0.005; *P < 0.02; Two-tailed; Unpaired t test in A–C and F–G. Circles within box plots represent each replicate.
HIV infection mediated polyamine synthesis is dependent on ODC-1
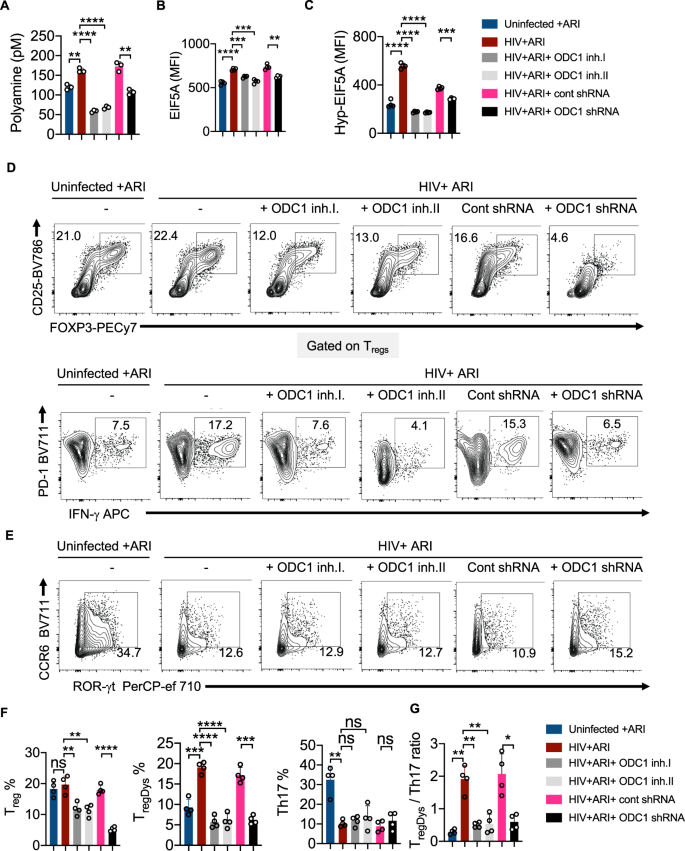
To further determine the role of ODC-1 in polyamine synthesis in CD4+ cells in the context of HIV infection, we performed the targeted assessment of specific polyamines in cells in the presence or absence of ODC-1 inhibition. We quantified putrescine, spermine, spermidine, cadaverine and thermospermine in cellular lysates using LC-MS as described in methods26. HIV-1 significantly upregulated putrescine levels both in the presence and absence of ARI. ARI partially reduced putrescine in HIV infected cultures (Fig. 6A). Spermidine and spermine were also significantly elevated during HIV infection, but ARI restored them almost to uninfected levels (Fig. 6B, C). Cadaverine was unaffected by HIV infection (Fig. 6D). Importantly, ODC-1 inhibition significantly diminished HIV-induced intracellular increases in putrescine, spermidine, and spermine, showing the critical function of ODC-1 in enhancing polyamine levels during HIV-1 infection (Fig. 6A–C).
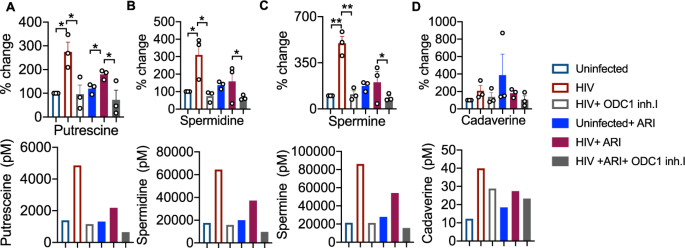
HTOC CD4+ T cells were TCR activated and infected with HIV. ARI and ODC-1 inhibitor I were added as indicated, 36 h post-infection. Quantification of putrescine (A), spermidine (B), spermine (C), and cadaverine (D) relative to the uninfected controls are shown (top). Thermospermine was not detected. Means with standard deviation error bars from three independent experiments are shown. n = 3 independent experiments. Unpaired t test one-tailed with Welch's correction; *P values 0.0126–0.0243, **P values 0.0038–0.0085 in A–D. Circles within box plots represent each replicate. Absolute levels (normalized to the input cell numbers) of the respective polyamines from one of the three independent experiments (bottom).
HIV-induced skewing of TregDys/Th17 ratios requires polyamine synthesis and exogenous polyamines cause Th dysregulation
We next determined whether polyamine enrichment and EIF5A hypusination could have contributed to HIV-mediated increase in TregDys/Th17 ratios. To corroborate the effect of polyamines and EIF5A hypusination independent of HIV-1 infection, we added exogenous putrescine hydrochloride, spermidine, and GC7, a DHPS inhibitor. Analogous to ODC-1 inhibition, GC7 also precipitously lessened the Treg and TregDys frequencies (Fig. 7A, top and bottom, first two panels in Fig. 7C), but did not restore Th17 depletion (Fig. 7B, 3rd panel in Fig. 7C) in HIV-infected HTOC CD4 cultures. While exogenous polyamines dramatically increased the frequency of TregDys, they did not temper the proportions of Th17 cells in uninfected cultures (Fig. 7A, B; last two panels and Fig. 7C). Although exogenous polyamines did not increase the TregDys/Th17 ratios as dramatically as HIV-1, they significantly increased them (Fig. 7C, last panel). Although exogenous polyamines did not increase Treg frequencies or lowered Th17 proportions, by simply increasing TregDys frequencies, they still significantly increased the TregDys/Th17 ratios. A closer examination of the viability of non Tregs, Tregs, and TregDys revealed that ODC-1 inhibition leads to cell death (Supplementary Fig. 14). However, ODC-1 inhibition and GC-7 caused significantly more cell death in non Tregs than in Tregs or TregDys. Similarly, spermidine also caused loss of viability in non Tregs but not in Tregs or TregDys. These data show that these inhibitors did not reduce TregDys by causing their cell death. Moreover, exogenous polyamines did not alter the viability of Tregs or TregDys, ruling out the possibility that polyamines increase the proportions of TregDys simply by increasing their viability. Because polyamines and GC7 have been implicated in autophagic cell death27, we further determined the expression of microtubule-associated proteins 1A/1B light chain 3B (LC3B), an autophagic protein in CD4+ T cells. While HIV infection, ARI, ODC-1 inhibitor I, or spermidine did not alter the protein levels, cell treated with GC7 showed elevated levels of LC3B (Supplementary Fig. 15). By using LY294002, an autophagic sequestration inhibitor, we further found that ODC-1inhibition and GC7 reduced TregDys proportions independently of autophagic cell death during HIV infection (Supplementary Fig. 16). These data highlighted the autophagic-independent effect of polyamines and EIF5A hypusination on TregDys cells and significantly heightening the TregDys/Th17 ratios in HTOC during HIV-1 infection.
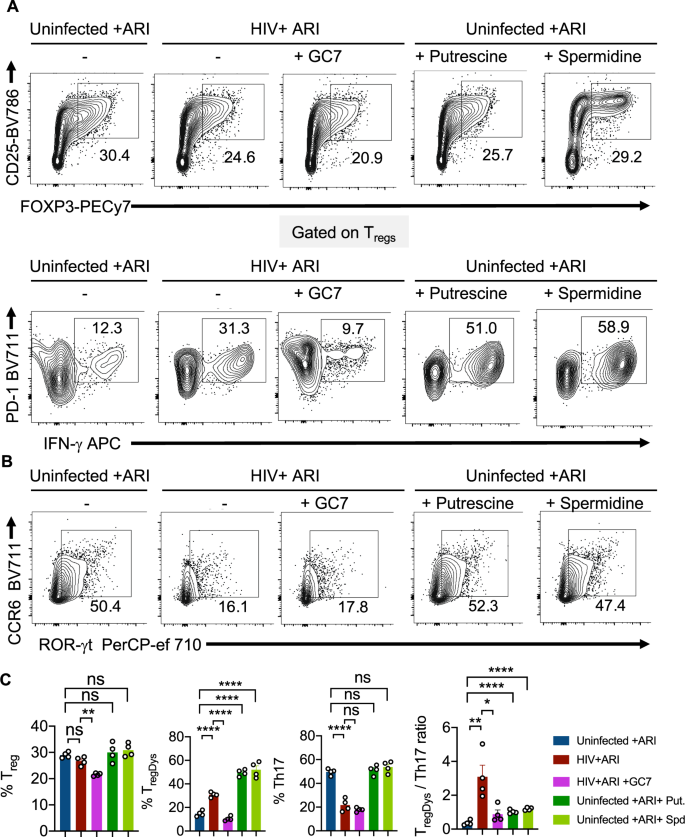
HTOC CD4+ T cells were stimulated as described in the methods. Some cultures were HIV-infected with or without GC7 (10 μM). Other uninfected cultures were treated with Putrescine dihydrochloride (100 μM) or Spermidine (1 mM). A Contour plots showing the percentage of Tregs (above) and PD-1hiIFN-γ+ cells in FOXP3+ population (TregDys) (below). B Contour plots showing ROR-γt and CCR6 expressing CD4+ T cells (Th17). C Quantification of TregDys/Th17 ratios as determined by flow cytometry analyses in A and B. Results represent four experiments with similar results and are presented as mean values +/− SEM. ****P < 0.0001; ***P = 0.0004; **P = 0.007; *P < 0.02; Two-tailed; Unpaired t test in C. Circles within box plots represent each replicate.
Exogenous addition of polyamines causes EIF5A upregulation, EIF5A hypusination leading to induction of TregDys cells and their proliferation
To further corroborate the effect of polyamines on Th cells, we evaluated the expression of ODC-1, EIF5A, and hypusinated-EIF5A in uninfected CD4+ T cells. Polyamines enhanced the expression of EIF5A, and hypusinated-EIF5A but significantly downmodulated ODC-1 expression in CD4+ T cells (Fig. 8A–C). To determine whether polyamines synthesized during HIV infection and exogenous polyamines induce FOXP3+PD-1+IFN-γ+TregDys or enhance IFN-γ in general in all CD4+ cells, we examined the expression of IFN-γ in non Treg CD4+ cells. As we showed previously, HIV-infection in fact reduced IFN-γ in non Treg CD4+ cells and ARI partially restored IFN-γ+ cells (Supplementary Fig. 17). Neither ODC-1 inhibition nor GC7 affected IFN-γ expression in non Tregs, showing that they reduced TregDys frequencies ...
Comments
Post a Comment